Most mammals, including humans, have two sex chromosomes, X and Y. One sex chromosome is usually inherited from each parent, and they pair up as either XX or XY in every cell of the body. People with XX chromosomes typically identify as female, and people with XY chromosomes typically identify as male. The genes on these chromosomes play a key role in development and function – including how heart disease develops.
Before I became a biomedical engineer studying how sex chromosomes affect the heart, I learned about one curious function of X chromosomes in my high school science class, with the calico cat example.
Female calico cats almost always have orange and black splotches of fur, because the gene that defines coat color is found on the X chromosome. When an orange cat mates with a black cat, female offspring, which typically inherit one X chromosome from each parent, will have a mixture of orange and black fur – one X chromosome encodes for orange fur while the other encodes for black fur. For this reason, male cats, which typically have one X and one Y chromosome, have solid orange or black coats.
Calico and tortoiseshell cats have multicolored patches of fur because only one of their two X chromosomes is activated in each cell.
How does this sex difference in fur color happen biologically? As it turns out, cells with XX chromosomes experience X-inactivation: The X chromosome from one parent is turned off in some cells, while the X chromosome inherited from the other parent is turned off in others. In the cells of female calico cats, X-inactivation can lead to splotches of orange and black fur if one X chromosome comes from a parent with orange fur and the other X chromosome comes from a parent with black fur.
X-inactivation happens because organisms like cats and people need only one X chromosome to function properly. To ensure the correct “dosage,” one of the X chromosomes is turned off in every cell. But some of the genes on the inactivated X chromosome escape inactivation and stay turned on. In fact, up to one-third of the genes on the X chromosome in people can escape inactivation, and they are thought to play a role in regulating health and disease.
Because X-inactivation happens only in those people with more than one X chromosome, researchers like me have been looking at how the genes that escape inactivation on the second X affect the health of people with XX chromosomes. We’ve found that for certain conditions, cell sex may be at the heart of the matter.
A change of heart
One disease that X chromosome escape genes partially regulate is aortic valve stenosis, a condition in which the part of the heart that controls blood flow to the rest of the body stiffens and narrows. This makes the heart work harder to pump blood and can ultimately lead to heart failure. Much like a person trying to push open a door with rusty hinges, the heart gets tired. There are currently no effective drugs available to slow or halt AVS disease symptoms.
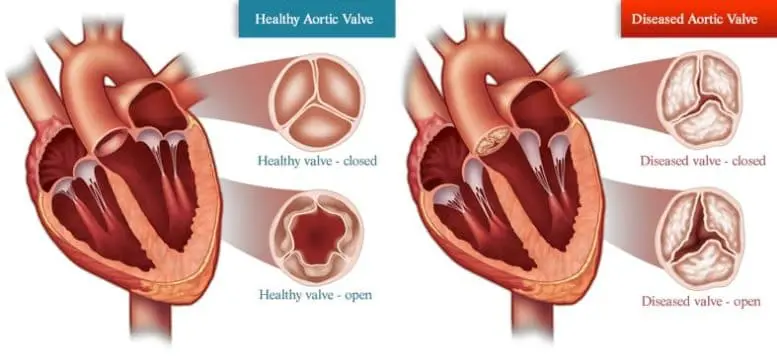
Hearts with aortic valve stenosis must pump harder to push blood through a narrowed aortic valve to the rest of the body. Credit: SuneErichsen/Wikimedia Commons, CC BY-SA
By and large, researchers think sex hormones drive sex differences in valve tissue stiffening. Indeed, decreasing estrogen levels during menopause can exacerbate heart fibrosis. However, studies on cardiovascular disease in XX and XY mice have found that sex differences still persist even after surgically excising the reproductive organs that produce sex hormones.
My team and I hypothesized that the genes that escape X-inactivation, being unique to people with XX chromosomes, may be driving these differences in valve stiffening. To test this idea, we developed bioengineered models of valve tissue using hydrogels. Hydrogels mimic the stiffness of valve tissue better than the traditional petri dish medium, allowing us to study heart cells in an environment that more closely resembles the body.
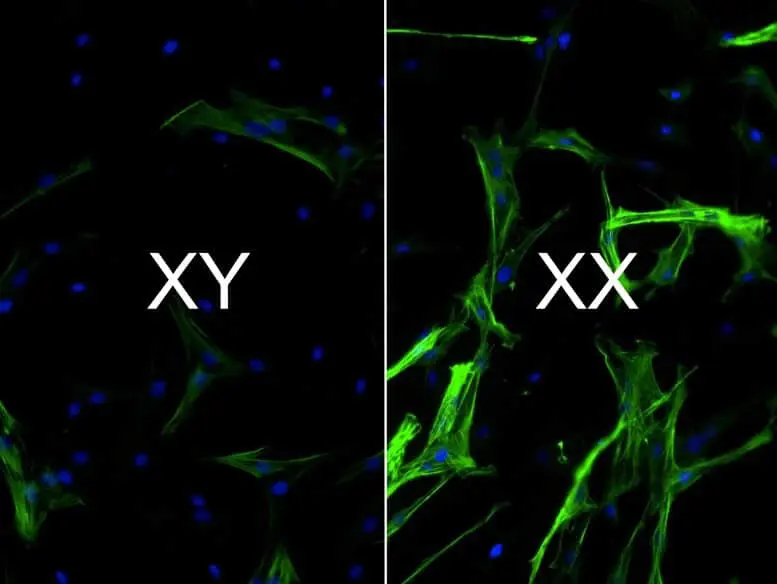
Heart tissue with XX chromosomes has a higher concentration of cells (colored green, with blue nuclei) that promote scarring than do cells with XY chromosomes. Credit: Brian Aguado, CC BY-NC-ND
We found that the cells we grew on our hydrogel models were able to replicate the sex differences seen in valve tissue – namely, valve cells with XX chromosomes had more scarring than cells with XY chromosomes. Moreover, when we decreased the activity of genes that escaped X-inactivation, we were able to decrease scarring in XX chromosome cells.
Our next step was to use our models to determine which treatments work best for AVS based on cell sex. We found that XX valve cells were less sensitive than XY cells to these drugs that targeted genes that promote scarring. Drugs that specifically target genes that escape X-inactivation, however, have a stronger effect on XX cells.
Equitable care for all
Sex and gender disparities in cardiovascular disease are rampant. For example, women are less likely than men to be prescribed cardiovascular medications despite guideline recommendations, and transgender individuals have higher rates of heart attacks than do cisgender folks.
Our work takes one more step toward achieving equity in developing medical therapeutics for cardiovascular disease. By taking sex chromosomes into consideration, my team and I believe that treatment strategies can be optimized for everyone, irrespective of cell “seXX.”
Written by Brian Aguado, Assistant Professor, University of California San Diego.
This article was first published in The Conversation.






