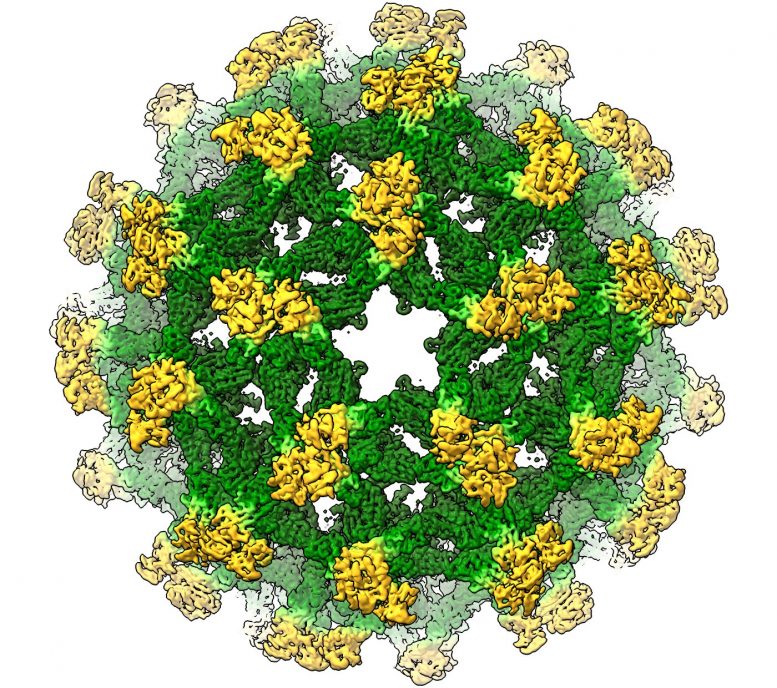
Cryo-electron microscopy reconstruction of Binjari virus. The projecting spikes are a typical feature of immature flaviviruses such as dengue virus but reveal an unexpected organization. Credit:
Associate Professor Fasseli Coulibaly
Better designed vaccines for insect-spread viruses like dengue and Zika now possible.
Better designed vaccines for insect-spread viruses like dengue and Zika are likely after researchers discovered models of immature flavivirus particles were originally misinterpreted.
Researchers from The University of Queensland and Monash University have now determined the first complete 3D molecular structure of the immature flavivirus, revealing an unexpected organization.
UQ researcher Associate Professor Daniel Watterson said the team was studying the insect-specific Binjari virus when they made the discovery.
“We were using Australia’s safe-to-handle Binjari virus, which we combine with more dangerous viral genes to make safer and more effective vaccines,” Dr. Watterson said.
“But when analyzing Binjari we could clearly see that the molecular structure we’ve all been working from since 2008 wasn’t quite correct.
“Imagine trying to build a house when your blueprints are wrong – that’s exactly what it’s like when you’re attempting to build effective vaccines and treatments and your molecular ‘map’ is not quite right.”
The team used a technique known as cryogenic electron microscopy to image the virus, generating high-resolution data from Monash’s Ramaciotti Centre for Cryo-Electron Microscopy facility.
With thousands of collected two-dimensional images of the virus, the researchers then combined them using a high-performance computing platform called ‘MASSIVE’ to construct a high-resolution 3D structure.
Monash’s Associate Professor Fasséli Coulibaly, a co-leader of the study, said the revelation could lead to new and better vaccines for flaviviruses, which have a huge disease burden globally.
“Flaviviruses are globally distributed and dengue virus alone infects around 400 million people annually,” Dr. Coulibaly said.
“They cause a spectrum of potentially severe diseases including hepatitis, vascular shock syndrome, encephalitis, acute flaccid paralysis, congenital abnormalities, and fetal death.
“This structure defines the exact wiring of the immature virus before it becomes infectious, and we now have a better understanding of the levers and pulleys involved in viral assembly.
“This is a continuation of fundamental research by us and others and, without this hard-won basic knowledge, we wouldn’t have the solid foundation needed to design tomorrow’s treatments.”
Reference: “The structure of an infectious immature flavivirus redefines viral architecture and maturation” by Natalee D. Newton, Joshua M. Hardy, Naphak Modhiran, Leon E. Hugo, Alberto A. Amarilla, Summa Bibby, Hariprasad Venugopal, Jessica J. Harrison, Renee J. Traves,§, Roy A. Hall, Jody Hobson-Peters, Fasséli Coulibaly and Daniel Watterson, 14 May 2021, Science Advances.
DOI: 10.1126/sciadv.abe4507
The joint first authors are Dr. Natalee Newton from UQ’s Watterson lab and Dr. Joshua Hardy from Coulibaly lab at the Monash Biomedicine Discovery Institute.





