Artistic rendering of dying cells protecting their neighbors to maintain tissue integrity. Holes in epithelium created by uncoordinated cell death are shown in purple. Credit: © Institut Pasteur / Léo Valon et Romain Levayer
Cells undergoing cell death protect their neighbors to maintain tissue integrity.
To enable tissue renewal, human tissues constantly eliminate millions of cells, without jeopardizing tissue integrity, form, and connectivity. The mechanisms involved in maintaining this integrity remain unknown. Scientists from the Institut Pasteur and the CNRS recently revealed a new process that allows eliminated cells to temporarily protect their neighbors from cell death, thereby maintaining tissue integrity. This protective mechanism is vital, and if disrupted can lead to a temporary loss of connectivity. The scientists observed that when the mechanism is deactivated, the simultaneous elimination of several neighboring cells compromises tissue integrity. This lack of integrity could be responsible for chronic inflammation. The results of the research were published in the journal Developmental Cell on June 2, 2021.
Human epithelia are tissues found in several parts of the body (such as the epidermis and internal mucosa). They are composed of layers of contiguous cells that serve as a physical and chemical barrier. This role is constantly being put to the test by both the outside environment and their own renewal. Tissue renewal involves the formation of new cells by cell division and the elimination of dead cells. The mechanisms that regulate the ability of epithelia to maintain their integrity in contexts involving large numbers of eliminated cells remain poorly understood, despite the fact that this situation occurs regularly during embryogenesis or the maintenance of adult tissues. For example, more than ten billion cells can be eliminated every day in an adult intestine. How are these eliminations orchestrated to maintain tissue integrity and connectivity?
Scientists from the Institut Pasteur and the CNRS set out to identify the mechanisms involved in epithelial integrity and the conditions that can affect epithelial connectivity by using Drosophila (or vinegar flies), an organism studied in the laboratory with a similar epithelial architecture to humans.
Using protein-sensitive fluorescent markers, the research team revealed that when a cell dies, the EGFR-ERK pathway – a cell activation signaling pathway known for its involvement in the regulation of cell survival – is temporarily activated in the neighboring cells. The scientists observed that the activation of the EGFR-ERK pathway protected neighboring cells from cell death for approximately one hour, thereby preventing the simultaneous elimination of a group of cells. “We already knew that this pathway plays a key role in regulating cell survival in epithelial tissue, but we were surprised to observe such protective dynamics between cells,” comments Romain Levayer, Head of the Cell Death and Epithelial Homeostasis Unit at the Institut Pasteur and last author of the study.
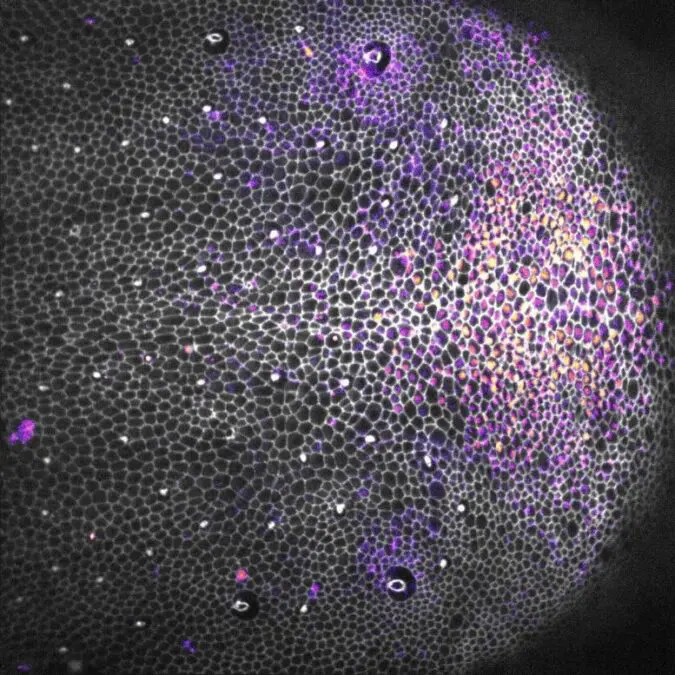
A Drosophila pupa epithelium showing cell contours (gray) and the reporter of the EGFR-ERK pathway (yellow/purple gradient). Credit: © Institut Pasteur / Romain Levayer et Léo Valon
The scientists’ research also shows that inhibiting this protective mechanism has a drastic effect on epithelial tissue: cell elimination becomes random and neighboring cells can be eliminated simultaneously, leading to repeated losses of connectivity. The elimination of groups of neighboring cells is never observed in epithelial tissue in normal conditions, when the EGFR-ERK pathway is not deliberately inhibited, even if a large number of cells are eliminated.
By using a new optogenetic tool that can control cell death in time and space and bypass the protective mechanism, the scientists confirmed that epithelial integrity was compromised when neighboring cells were eliminated simultaneously. “Surprisingly, epithelial tissue is highly sensitive to the spatial distribution of eliminated cells. Although it can withstand the elimination of a large number of cells, epithelial integrity is affected if just three neighboring cells are eliminated simultaneously,” explains Léo Valon, a scientist in the Cell Death and Epithelial Homeostasis Unit at the Institut Pasteur and first author of the study.
The scientists’ observations confirm that tissues need to develop mechanisms preventing the elimination of neighboring groups of cells. “These observations are important as they illustrate the incredible self-organizing ability of biological tissues, a property that enables them to withstand stressful conditions. So there is no need for a conductor to orchestrate where and when the cells should die; everything is based on highly local communications between neighboring cells,” adds Romain Levayer.
This process seems to have been conserved during evolution. The same protective mechanism based on local EGFR-ERK activation was discovered independently in human cell lines by the research group led by Olivier Pertz at the University of Bern in Switzerland (the results are published in the same journal2). The results of the other study suggest that the protective mechanism is conserved between species separated by hundreds of millions of years, indicating that it is a relatively universal mechanism.
Future research will reveal whether disruption to this cell death coordination mechanism and repeated loss of connectivity in epithelial tissue could be one of the roots of chronic inflammation, a phenomenon responsible for various diseases that are currently among the leading causes of death worldwide.
Distribution of cell deaths in a Drosophila epithelium:
Development of the Drosophila pupa epithelium showing the location of all cell deaths (colored dots). The cell contours are shown in gray. Credit: © Institut Pasteur / Léo Valon et Romain Levayer
Activation of the EGFR-ERK pathway in neighboring cells:
Activation of the EGFR-ERK pathway in the neighbors of a cell extruded from the tissue. The reporter on the left is excluded from the nucleus when the pathway is activated (the eliminated cell is circled in green). Activation can also be viewed by other pathway sensors (the FRET sensor – red for strong activation. Credit: © Institut Pasteur / Romain Levayer et Léo Valon
References:
- “Robustness of epithelial sealing is an emerging property of local ERK feedback driven by cell elimination” by Léo Valon, Anđela Davidović, Florence Levillayer, Alexis Villars, Mathilde Chouly, Fabiana Cerqueira-Campos and Romain Levayer, 2 June 2021, Developmental Cell.
DOI: 10.1016/j.devcel.2021.05.006 - “Collective ERK/Akt activity waves orchestrate epithelial homeostasis by driving apoptosis-induced survival” by Paolo Armando Gagliardi, Maciej Dobrzyński, Marc-Antoine Jacques, Coralie Dessauges, Pascal Ender, Yannick Blum, Robert M. Hughes, Andrew R. Cohen and Olivier Pertz, 2 June 2021, Developmental Cell.
DOI: 10.1016/j.devcel.2021.05.007
This research project was supported by the European Research Council (ERC), a Marie Skłodowska-Curie post-doctoral fellowship, the Fondation pour la Recherche Médicale (FRM) et the Cercle Fondation Schlumberger pour l’Education et la Recherche (FSER), R.Levayer 2019 laureate.





